Solution Phase Scavenge and Capture Chemistry
|
|||
|
|
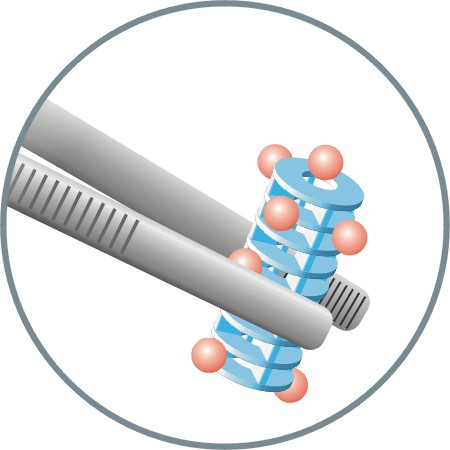
Chemists working in solution phase must sometimes resort to resin techniques as an aid to synthesis or purification. SynPhase Scavenger Lanterns can help transcend the inherent practical limitations here. A range of high loading functionalised surfaces are available for scavenging excess reagents from solution, or to facilitate capture and release techniques without any of the messy weighing or filtering processes used with conventional resins.
|
To enhance productivity, libraries of compounds prepared with solution phase techniques can make use of the various high-throughput tagging methods available for tracking and manipulating Lanterns in large numbers.
Scavenger Lanterns are suitable for a wide range of applications including scavenging of nucleophiles, electrophiles and various amines as well as solid supported reagents for amide bond formation.
Range of Functionalizations
SynPhase Scavenger Lanterns have been optimized for a variety of chemistries, as shown in the table below. The loading range of these Lanterns is significantly higher than other types, up to around 80umol to 150umol in the case of A-series Lanterns.
For ordering information, refer to Scavenger Lanterns in the SynPhase Catalog.
| Functionalization |
Chemistry application |
| Sulphonic Acid | Scavenges primary, secondary and tertiary amines by quarternary salt formation. |
| Aminomethyl | Scavenges acid chlorides, sulfonyl chlorides, isocyanates and other electrophiles. |
| TMI Isocyanate | Scavenges primary and secondary amines but does not scavenge anilinic type aromatic amines. |
| N-Methyl Morpholine | Supported tertiary amine for acylations and sulfonylations. |
| o-Nitrophenol | Solid supported active-ester used for synthesis of amides and sulfonamides. |
| Benzaldehyde | Scavenges various nucleophiles including amines, hydrazines and carbon-based nucleophiles such as phenylmagnesium halides. |
Chemical Performance
Solution phase chemists are accustomed to expecting high levels of purity. Whilst SynPhase Lanterns bring exceptional handling convenience to the workbench there is certainly no compromise in performance. Scavenging rates are comparable to or better than resin, and this is reflected in the high yield and purity of products. The following are specific examples:
Example 1) – Scavenging
100% consumption of benzylamine by sulfonic acid (SOH) Lanterns at room temperature takes less than 10 minutes.
Example 2) – Catch and Release
Solid bound chloronicotinyl active-ester on o-Nitrophenol (ONP) Lanterns reacts with furfurylamine in dichloromethane at room temperature in less than 6 hours to give 99% pure amide.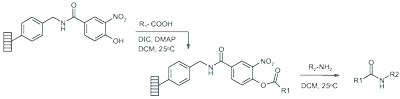
Further information on technical applications is available in SynPhase Technical Note SCN017.
Synthesis Strategies
Libraries of solution-phase compounds can be scavenged in parallel using the Multipin Array Format. With this approach, multiples of up to 96 SynPhase Lanterns are mounted onto Stems which in turn are placed on a Stem holder. SynPhase Combinatorial provides a suite of Assembly tools to facilitate expedient handling of Lanterns in large numbers, either individually or in blocks of 96.
The Multipin Array Strategy is also suitable for creating combinatorial libraries based on Catch and Release chemistry using Scavenger Lanterns. Colored tags provide an ideal method for optimising the synthetic conditions for these libraries.
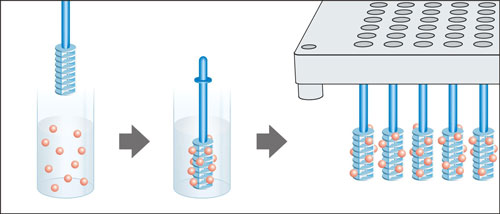
Lanterns mounted on Stems can scavenge solution phase products or unused reagents in parallel