Lanterns for Solid-Phase Organic Synthesis
These commonly used linkers are readily available on SynPhase Lanterns to perform a wide range of chemistries based on both the polystyrene and polyamide surface polymers
| Alkyl Tethered Diisopropylarylsilane | |
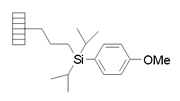 |
Chemistry applications: |
| Product: |
|
| PolymerTypes: (PS) Polystyrene |
| Backbone Amide |
|
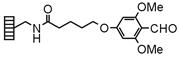 |
Chemistry applications: Backbone Amide Lanterns link amines via reductive amination. Acylation of the amine nitrogen affords secondary amides on cleavage. In an analogous fashion, sulfonamides and ureas can be generated. |
| Loading method: Amines: reductive amination in the presence of NaBH3CN under acidic conditions. | |
| Product: Secondary amides, sulfonamides and ureas. | |
| Cleavage: >20% TFA/DCM | |
| PolymerTypes: (PS) Polystyrene, (PA) Polyamide |
| Brominated | |
| Chemistry applications: |
|
| Product: |
|
| PolymerTypes: (PS) Polystyrene |
| Bromoacetal | |
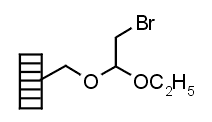 |
Chemistry applications: |
| Product: |
|
| PolymerTypes: (PS) Polystyrene |
| Hydroxymethylphenoxy | |
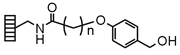 |
Chemistry applications: HMP Lanterns are derivatized with Mimotopes' modified HMP type Linker. HMP Lanterns link carboxylic acids, phenols and amines (as urethane derivatives). Carboxylic acids are normally coupled using DIC/N,Ndimethylaminopyridine (DMAP), and phenols via imidate or Mitsunobu chemistry. Cleavage by acidolysis affords the original functional group; carboxylic acid esters are also labile to strong base. |
| Loading method: Acids: DIC/DMAP coupling, Phenols: Imidate, Mitsunobu | |
| Cleavage: >20% TFA/DCM | |
| Product: Carboxylic acids, phenols |
|
| PolymerTypes: (PS) Polystyrene, (PA) Polyamide |
| Hyperlabile |
|
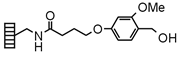 |
Chemistry applications: Hyperlabile Lanterns link carboxylic acids, phenols and amines (as urethane derivatives). Carboxylic acids are normally coupled using diisopropylcarbodiimide (DIC)/N,Ndimethylaminopyridine (DMAP), and phenols via imidate or Mitsunobu chemistry. Cleavage by acidolysis affords the original functional group; carboxylic acid esters are labile to strong base. |
| Product: Carboxylic acids, phenols |
|
| Loading method: Acids: DIC/DMAP coupling, Phenols: Imidate, Mitsunobu | |
| Cleavage: >1% TFA/DCM | |
| PolymerTypes: (PS) Polystyrene |
| Kaiser | |
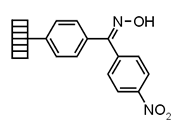 |
Chemistry applications: |
| Product: |
|
| PolymerTypes: (PS) Polystyrene |
| No Derivitization |
|
| Chemistry applications: |
|
| Product: |
|
| PolymerTypes: |
| Oxidized Hyperlabile |
|
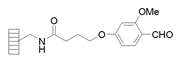 |
Chemistry applications: |
| Product: |
|
| PolymerTypes: (PS) Polystyrene |
| Rink Amide |
|
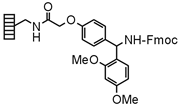 |
Chemistry applications: Rink Amide Linker can be loaded with activated carboxylic acids, which cleave to afford primary carboxamides. Sulfonyl chlorides afford primary sulfonamides on cleavage. This linker is also used for condensation reactions. RAM Lanterns are supplied in Fmoc protected form and require deprotection with piperidine prior to use. The RAM linker is stable to base but not to strong oxidants. |
| Product: Primary amides, primary sulfonamides |
|
| Loading method: Acylation with activated acids, sulfonylation. | |
| Cleavage: >20% TFA/DCM | |
| PolymerTypes: (PS) Polystyrene, (PA) Polyamide |
| Trityl Alcohol |
|
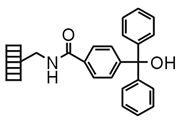 |
Chemistry applications: Trityl Alcohol Trityl alcohol Lanterns, after being converted to trityl chloride upon treatment with acetyl chloride, can be used to link carboxylic acids, alcohols, phenols and amines. Cleavage by acidolysis affords the original functional group. |
| Product: Phenols, heterocycles, acids, amines, thiols, etc. | |
| Loading method: Form trityl chloride; nucleophile/base | |
| Cleavage: 1% TFA/DCM | |
| PolymerTypes: (PS) Polystyrene, (PA) Polyamide |
| Wang | |
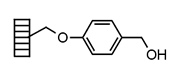 |
Chemistry applications: |
| Product: |
|
| PolymerTypes: (PS) Polystyrene |



